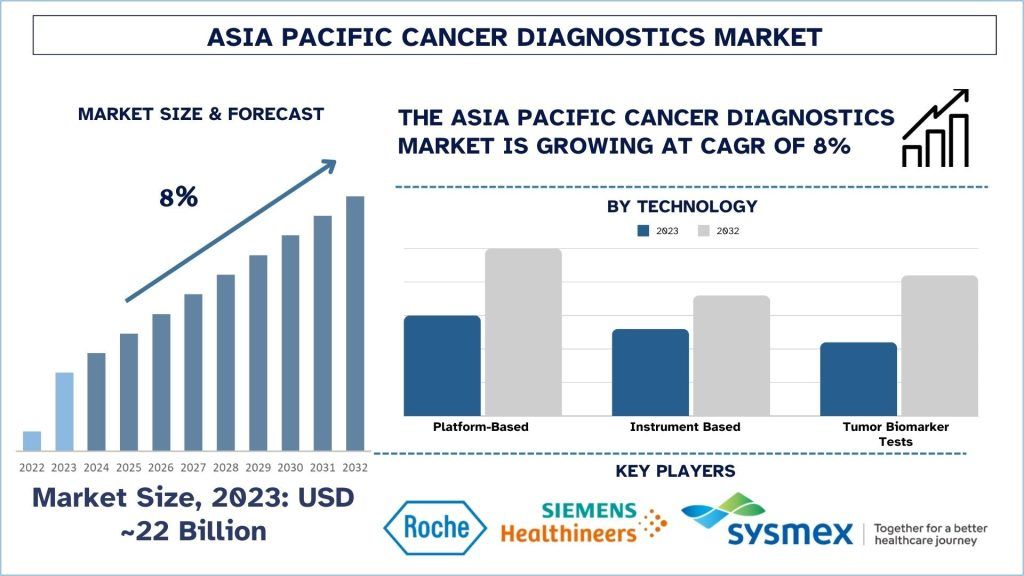
According to UnivDatos, the Asia Pacific Cancer Diagnostics Market was valued at approximately USD 22 billion in 2023 and is projected to grow at a strong CAGR of around 8% during the forecast period (2024–2032). This growth is primarily driven by the rising prevalence of cancer across Asia, along with continuous advancements in diagnostic technologies and improving access to healthcare services. The Asia-Pacific (APAC) cancer diagnostics landscape is undergoing rapid transformation due to innovation, increasing disease burden, and expanding healthcare infrastructure. Cancer diagnostics has become a critical segment of modern medical science, and the APAC region represents both a significant challenge and a major opportunity within the global healthcare ecosystem. Population growth, diverse healthcare needs, and strengthening healthcare systems are intensifying the demand for early and accurate cancer detection. This overview highlights the current market scenario, recent advancements, and strategic initiatives shaping the cancer diagnostics industry in the Asia-Pacific region.
Rising Incidence of Cancer Driving Market Demand
World Economic Forum, 2023
- Asia accounts for approximately 45% of global breast cancer cases and nearly 58% of cervical cancer-related deaths worldwide.
- Every USD 1 invested in cervical cancer elimination initiatives could generate economic returns exceeding USD 3.
- Regional governments have multiple actionable pathways to reduce the overall cancer burden among women.
Access sample report (including graphs, charts, and figures): https://univdatos.com/reports/asia-pacific-cancer-diagnostics-market?popup=report-enquiry
The increasing incidence of cancer remains one of the primary drivers of the APAC cancer diagnostics market. According to global health estimates, the region accounts for nearly half of all newly diagnosed cancer cases annually. This surge is linked to multiple risk factors such as aging populations, lifestyle changes, environmental pollution, tobacco use, and dietary habits. Common cancers in the region include lung, breast, colorectal, and stomach cancer, with lung cancer being particularly prevalent due to high smoking rates and worsening air quality.
The growing cancer burden has compelled healthcare systems across Asia-Pacific to prioritize early detection and preventive diagnostics. Governments, healthcare providers, and diagnostic companies are expanding cancer screening programs and investing in advanced diagnostic technologies to improve detection accuracy and reduce mortality rates. Early diagnosis not only enhances patient prognosis but also significantly lowers long-term treatment costs and healthcare system strain.
Technological Advancements: AI and Genomics
On September 9, 2024, Roche announced the expansion of its digital pathology open ecosystem through the integration of over 20 advanced artificial intelligence (AI) algorithms developed in collaboration with eight new partners. These collaborations aim to support pathologists and researchers in improving cancer diagnosis and research outcomes through AI-powered insights.
In July 2024, researchers at Shanghai Jiao Tong University, China, introduced an AI-based laboratory test capable of detecting three cancer types—colorectal, gastric, and pancreatic—from a single drop of dried blood. This paper-based diagnostic test successfully differentiated cancer patients from non-cancer individuals, demonstrating strong potential for early, low-cost screening.
Technological innovation continues to play a pivotal role in transforming cancer diagnostics across the Asia-Pacific region. The adoption of AI-driven diagnostic tools is accelerating, particularly in medical imaging and pathology, where algorithms assist in identifying cancerous lesions, analyzing tumor characteristics, and predicting disease risk. These technologies significantly enhance diagnostic precision while reducing turnaround time, enabling faster initiation of treatment.
Genomic testing is another rapidly expanding segment within the APAC cancer diagnostics market. With the growing focus on precision and personalized medicine, genomics is becoming essential for identifying genetic mutations and biomarkers associated with various cancers. Advanced techniques such as next-generation sequencing (NGS) are increasingly used to guide targeted therapies based on individual patient profiles.
Liquid Biopsy: Transforming Cancer Diagnostics
Liquid biopsy has emerged as a revolutionary diagnostic approach in cancer detection across the Asia-Pacific region. Unlike traditional tissue biopsies, liquid biopsies analyze circulating tumor DNA or cancer cells from blood samples, offering a non-invasive and patient-friendly alternative. This technology enables early cancer detection, real-time monitoring of treatment response, and identification of disease recurrence.
The adoption of liquid biopsy is gaining momentum in APAC, particularly for lung cancer diagnostics, given its high prevalence and mortality rate in the region. Leading companies such as Illumina, QIAGEN, and Roche are actively developing liquid biopsy solutions tailored to regional healthcare needs and regulatory frameworks.
Increasing Investments and Strategic Collaborations
Rising investments and collaborations between global and regional players are significantly fueling growth in the Asia-Pacific cancer diagnostics market. Major multinational companies such as Roche, Abbott Laboratories, and Siemens Healthineers are strengthening their presence in the region through partnerships with healthcare institutions, governments, and local firms. These alliances aim to improve access to advanced diagnostic technologies and address country-specific healthcare challenges.
Click here to view the Report Description & TOC: https://univdatos.com/reports/asia-pacific-cancer-diagnostics-market
On August 6, 2024, Sysmex Corporation announced the expansion of its strategic alliance with QIAGEN N.V., enhancing collaboration across research and development, manufacturing, clinical validation, and sales and marketing of genetic testing solutions.
China has emerged as a leading destination for cancer diagnostics investments, supported by government initiatives such as the Healthy China 2030 program. This initiative has created a favorable environment for both domestic and international players to expand diagnostic capabilities. Strategic partnerships are helping companies navigate regulatory pathways, improve technology adoption, and expand market reach.
India also represents a high-growth market within APAC due to rising healthcare awareness and increasing emphasis on early cancer diagnosis. Domestic companies are developing affordable diagnostic solutions to cater to the large population, while international firms are entering the market through joint ventures and collaborations.
Regulatory Framework and Government Support
Regulatory policies across Asia-Pacific play a critical role in shaping the cancer diagnostics market. Countries such as Japan, Australia, and South Korea maintain well-established regulatory bodies to ensure diagnostic safety and efficacy. However, stringent regulatory requirements can sometimes delay product launches, particularly in emerging economies.
To address this, several governments are streamlining approval processes. In China, the National Medical Products Administration (NMPA) has introduced measures to accelerate the approval of innovative diagnostic technologies. Similarly, India’s Central Drugs Standard Control Organization (CDSCO) has implemented reforms aimed at faster market entry for diagnostic products.
Governments across the region are also actively promoting national cancer screening programs. Japan operates comprehensive screening initiatives for breast, cervical, and colorectal cancers, while China has intensified lung cancer screening efforts in response to high smoking prevalence.
Future Outlook
The future of cancer diagnostics in the Asia-Pacific region appears highly promising. Continuous technological advancements, growing awareness of early detection, and strong collaboration between governments, healthcare providers, and private companies are shaping a robust diagnostic ecosystem. Innovation, affordability, and accessibility will remain key priorities to ensure advanced diagnostic solutions reach broader populations.
In conclusion, the Asia-Pacific cancer diagnostics market is poised for transformative growth, driven by the rising cancer burden, rapid technological progress, and increasing investments. With a growing emphasis on early diagnosis and personalized treatment, the region is expected to play a leading role in advancing global cancer care.
Contact Us:
UnivDatos
Contact Number – +1 978 733 0253
Email – contact@univdatos.com
Website – www.univdatos.com
Linkedin- https://www.linkedin.com/company/univ-datos-market-insight/mycompany/